Iron can be obtained by reduction of iron oxide (Fe3O4) with CO according to the reaction: Fe3O4 + 4CO → 3Fe + 4CO2 How many kg of Fe3O4 should be heated with

Nanomaterials | Free Full-Text | Superparamagnetic Fe3O4@CA Nanoparticles and Their Potential as Draw Solution Agents in Forward Osmosis

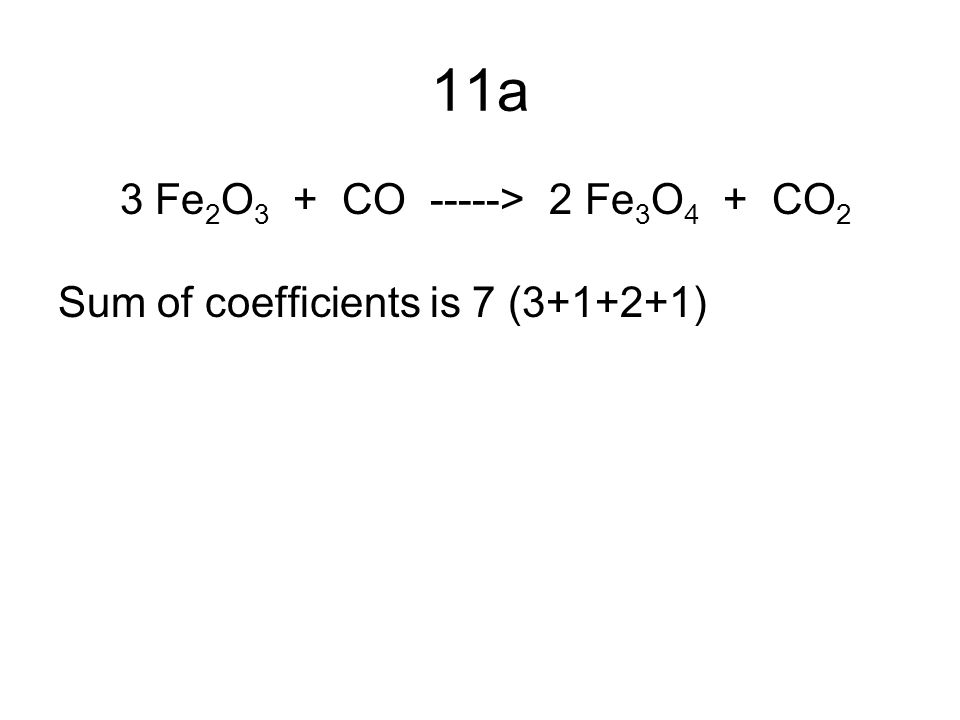
SOLVED: Question 29 (2 points) Which of these choices shows the following chemical reaction properly balanced? FezO3 + CO Fe3O4 CO2 FezO3 2CO Fe3O4 2C02 3FezO3 + CO 2Fe3O4 + CO2 None

Balance the following equations a Fe H2O Fe3O4 H2 b Ca N2 Ca3N2 c Zn KOH K2ZnO2 H2 d Fe2O3 CO Fe CO2...

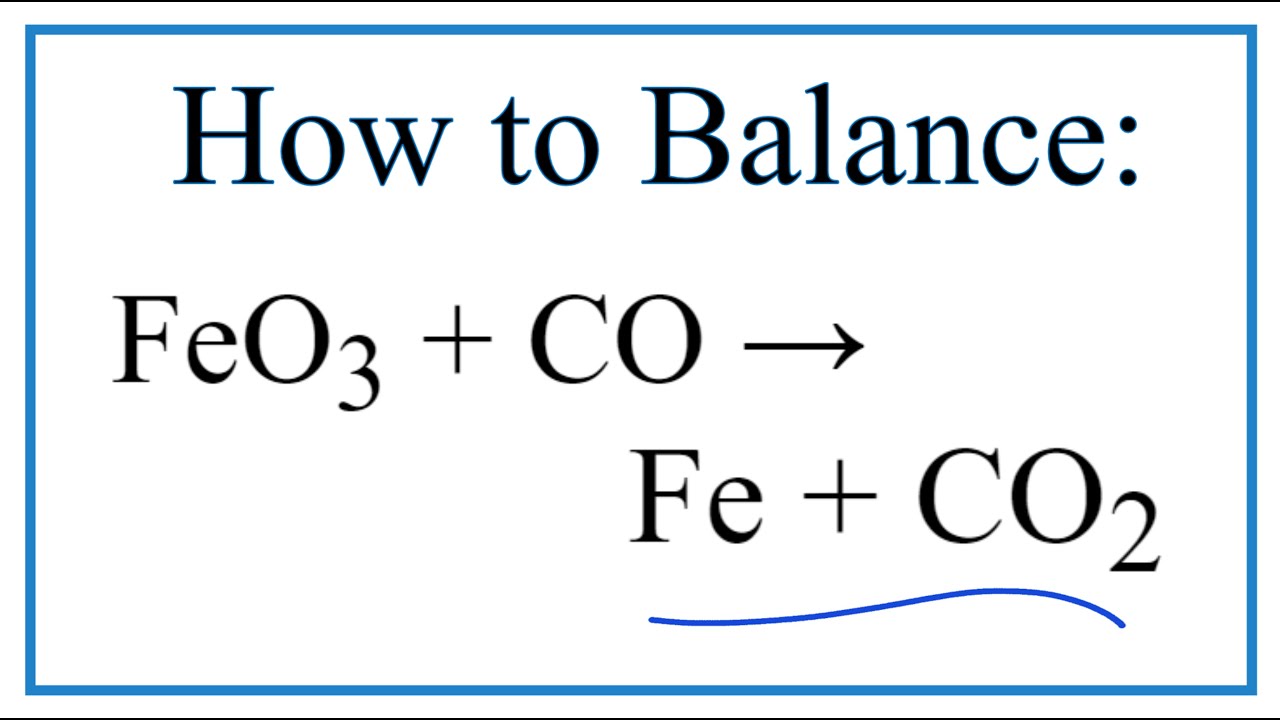
Balance this equation Fe2O3 + CO - Fe + CO2 - Chemistry - Atoms and Molecules - 16128049 | Meritnation.com

An Atomic Insight into the Confusion on the Activity of Fe3O4 Nanoparticles as Peroxidase Mimetics and Their Comparison with Horseradish Peroxidase | The Journal of Physical Chemistry Letters

Life Cycle Impact Assessment of Iron Oxide (Fe3O4/γ-Fe2O3) Nanoparticle Synthesis Routes | ACS Sustainable Chemistry & Engineering

The effect of Fe3O4 nanoparticles on the mass transfer of CO2 absorption into aqueous ammonia solutions - ScienceDirect
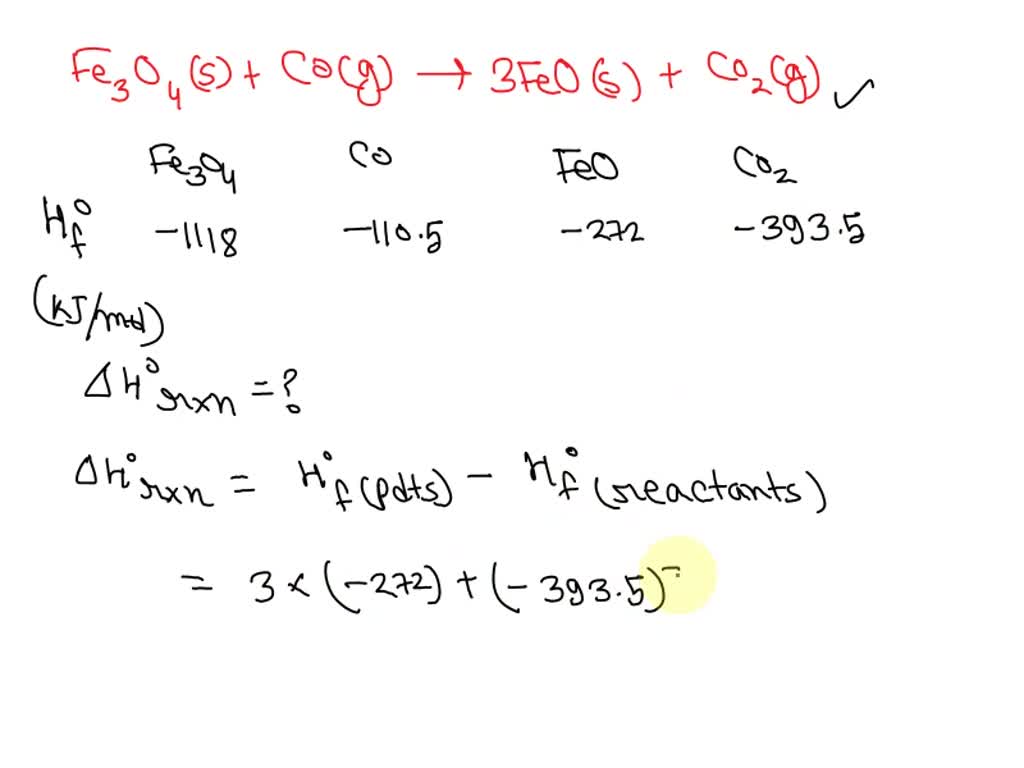
SOLVED: Calculate Horxn for the following reaction at 25.0oC: Fe3O4(s) + CO(g) –> 3FeO(s) + CO2(g) Hfo (kJ/mol) => Fe3O4(s) = -1118, CO(g) = -110.5, FeO(s) = -272, CO2(g) = -393.5

High Conversion to Aromatics via CO2-FT over a CO-Reduced Cu-Fe2O3 Catalyst Integrated with HZSM-5 | ACS Catalysis
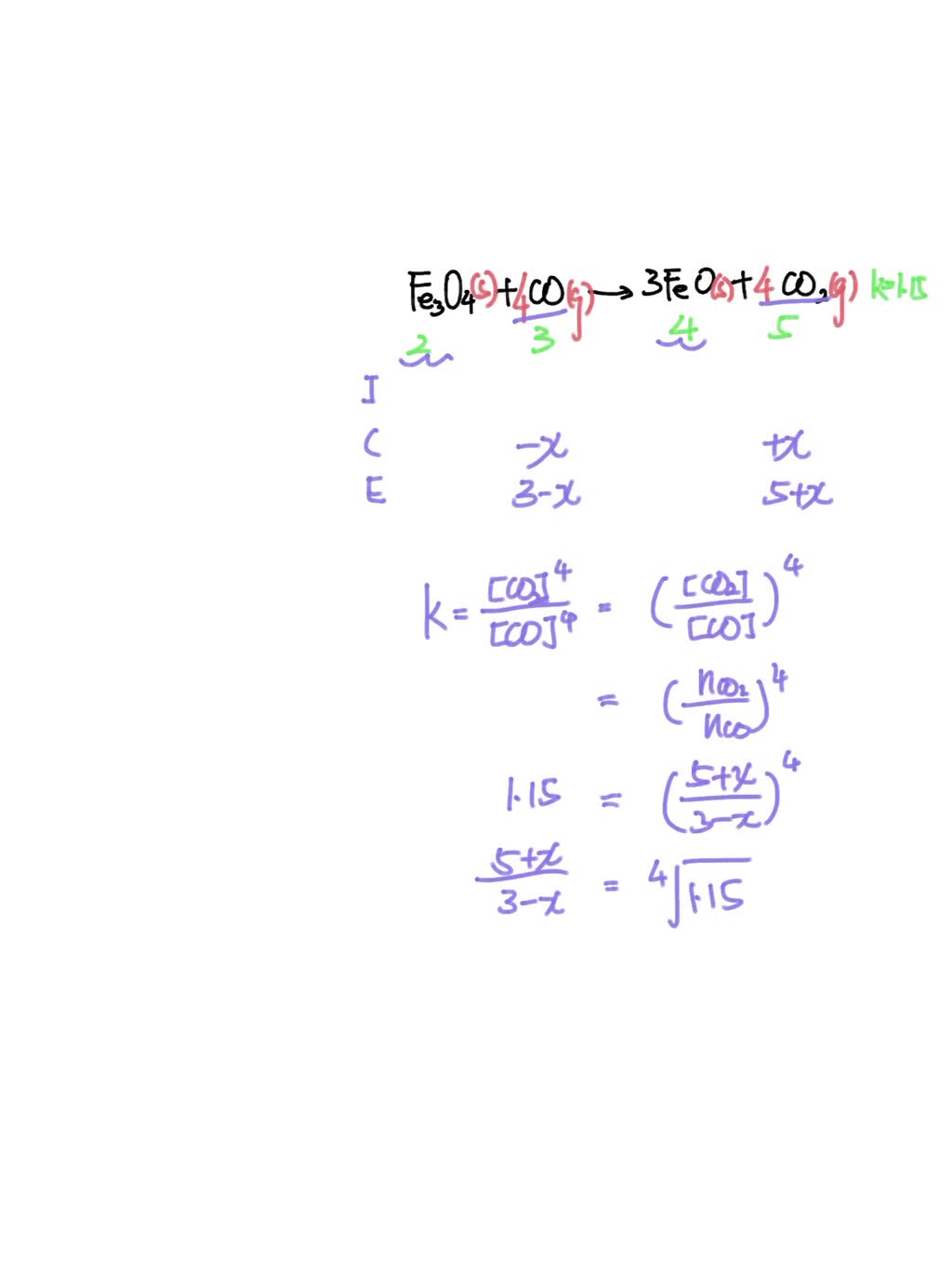
SOLVED: The equilibrium constant for the reaction Fe3O4 (s) + CO (g) ↔ 3FeO (s) + CO2 (g) It is 1.15 at 600 ° C. If a mixture of 2.00 moles of



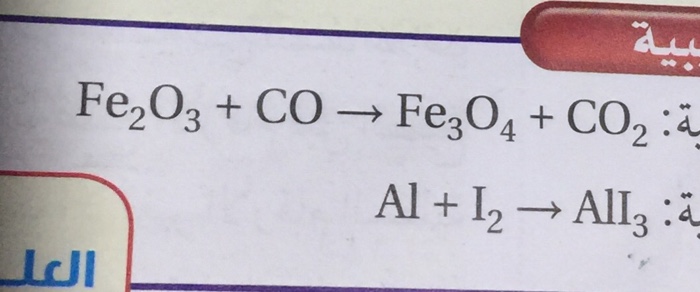
![ANSWERED] Iron oxide ores, commonly a mixture of F... - Inorganic Chemistry ANSWERED] Iron oxide ores, commonly a mixture of F... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/50670046-1658929430.9444451.jpeg)